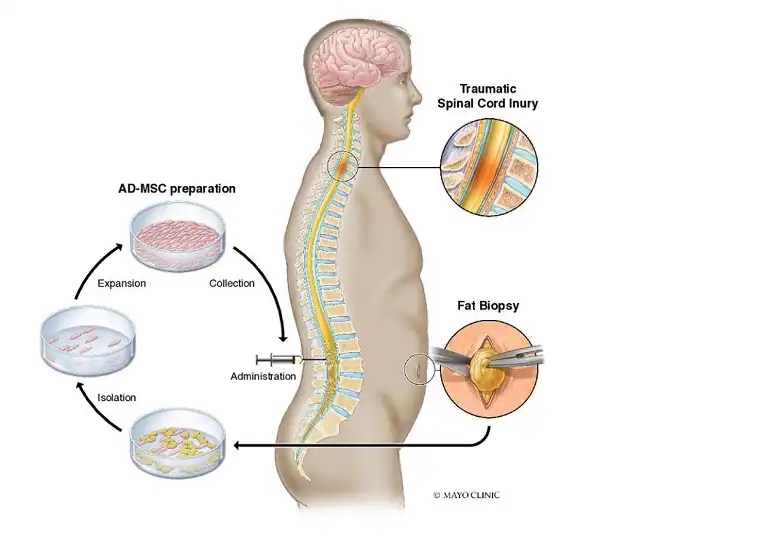
Regenerative cure focuses on repairing function by allowing the body to fix itself. At Stemcellcure & Eyestemcellcenter, mesenchymal stem cells (MSCs) are at the center of this approach. These adult stem cells—typically come from cartilage marrow, fatty (fat) tissue, or umbilical cord fabric—are revered for their ability to calm swelling, support tissue repair, and advance curative through bioactive signals.
The article below is about how Stemcellcure Uses Mesenchymal Stem Cells (MSCs) for Regenerative Medicine.
Why MSCs?
MSCs offer an alterable, clinically efficient alternative cause they:
• Modulate privilege:
They can wheel down extravagant active invulnerable responses that drive chronic lump.
• Support adjustment:
In the right microenvironment, MSCs can differentiate toward cartilage, cartilage, bone, and supplementary mesenchymal lineages.
The Stemcellcure Care Pathway
1. Comprehensive Assessment
Patients undergo a detailed conference, imaging review, and testing room screening. The goal search out and confirm the likely benefit of an MSC approach and to design an agreement that complements existing therapies, not replaces them without excuse.
2. Source Selection & Harvest
Depending on the indication and patient factors, MSCs can be obtained from:
- Bone essence aspirate (BMA)
- Adipose tissue via minimally obtrusive lipoharvest
- Allogeneic umbilical rope tissue (where managing permit and donor protect/traceability are robust)
3. Processing & Quality Controls
Stemcellcure uses controlled workshop workflows to concentrate, separate, or expand MSCs as allowed by local management. Rigorous quality checks—cell identity, viability, sterility, and dose—help ensure production consistency and safety.
4. Personalized Delivery
Route and drug are tailored to the condition:
- Intravenous administration when systemic immunomodulation is wanted
- Combination protocols making local and systemic delivery
Stemcellcure emphasizes informed consent, sensible expectations, and devotion to applicable regulations and directions. Donor screening (for allogeneic commodity), chain-of-custody documentation, and unfavorable-event listening are integral to every obligation. Patients are clearly counseled that outcomes vary that MSC therapy is not a protected cure.
Data-Based Therapy
Clinical evidence for MSCs is strongest in select orthopedic and inflammatory environments, with continuous trials exploring more extensive applications. Stemcellcure integrates:
- Published research to guide contract design,
- Objective measures (pain scores, functional scales, imaging) to track progress,
- Continuous improvement established real-world consequences and a safety dossier.
What Patients Can Expect
• Clear goals:
Pain reduction, enhanced mobility, faster improvement from soft-tissue harms, or better quality of history where conventional alternatives are limited.
• Minimal free time:
Many procedures are outpatient, accompanied by brief recovery windows.
• Stepwise plans:
If appropriate, MSC therapy can be produced alongside PRP, hyaluronic acid, or standard care.
Conclusion
Stemcellcure’s MSC programs combine patient collection, high-standard cell alteration, precision transfer, and structured rehabilitation to present the body allure best chance to repair.
For individuals seeking progressive, evidence-informed educational options—especially for musculoskeletal and inflammatory environments—MSCs provide a hopeful, carefully managed road. Patients are encouraged to examine personal risks, benefits, costs, and alternatives accompanying the clinical group to make a fully cognizant decision.